- Where and how water molecules form in the Universe
- What heavy water is
- How heavy water traces the cosmic history of water
Have you ever heard the old adage that the water you drink is likely to contain water molecules that passed through the dinosaurs? It’s crazy to think that the water we drink could have been around that long. What’s even crazier is that the molecules of water on Earth may actually be a lot older than the age of the Earth, or even the Sun!
The origin of Earth’s water and, hence, how old it actually is, is one of the biggest open questions in astronomy. Water molecules originally form in cold interstellar clouds of dust and gas where stars form. But is this pristine water the same that we’re drinking today, or have these molecules been destroyed and reformed on their journey here? Researchers have now found a new piece of this puzzle.
To understand how old the water in our little rock is, we first need to start where the water journey also begins…

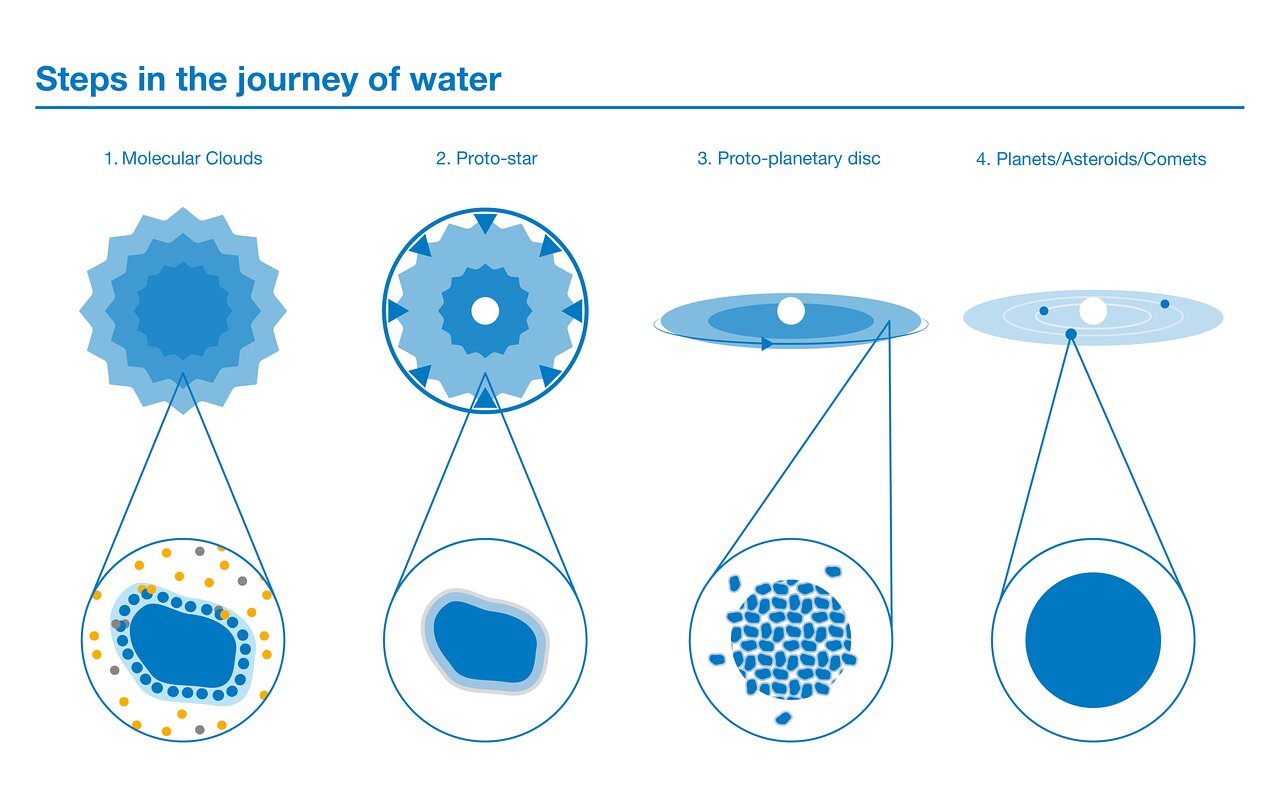
Tiny droplets in an ocean of matter
In the cold, dense clumps of gas and dust that form molecular clouds, the first step of water creation occurs. Oxygen atoms sit on grains of dust within the cloud, and interact with free-floating hydrogen atoms to form water molecules. The water that is formed isn’t liquid, but remains frozen on the surface of the dust grains.
In the next phase gravity slowly takes over and the clump of molecular gas gets denser and denser until it collapses, forming a ‘proto-star’. A lot of heat is generated from this collapse and further transferred to the surrounding material. As the dust grains heat up in the central parts of the cloud, the frozen water that coats them sublimates, instantly turning to gas without first becoming liquid. But further out, the bulk of the water reservoir remains frozen. This water ice is harder to detect than gaseous water, but it’s also less prone to being altered by chemical processes. Water thus becomes the second most abundant molecule (after molecular hydrogen) in this warm cloud around the baby star, which contains 10 000 times more water than the oceans on Earth combined.
As the matter in the central parts of the cloud collapses, it starts to spin faster and flattens out into a disc around the proto-star. Most of the water in this ‘proto-planetary disc’ remains frozen around dust grains at this stage. Dust grains are in that sense something like the Guardians of the Water History.
Comets are the next step in the journey of water. These icy bodies form out of dust grains in the proto-planetary disc, and they can potentially deliver water to planets. But how can we be sure that water molecules in comets are chemically the same as those originally formed in the parent cloud? The way to trace the path of water is through something called heavy water.
What’s heavy water and where does it come from?
Just like normal water, heavy water comprises an oxygen atom and two hydrogen atoms. But the hydrogen in heavy water is not really hydrogen: it’s deuterium, a heavier isotope of hydrogen with one proton and one neutron instead of just one proton, hence its name.
The puzzling thing is that deuterium is only produced in the first few seconds after the Big Bang, and in very small amounts: there are about 100 000 times more normal hydrogen atoms than deuterium ones. If this fixed abundance of deuterium were to be uniformly distributed in water molecules, the expected ratio of heavy to normal water would be much lower than what we actually observe in the Solar System today.
It turns out that there are many more deuterium atoms on the surfaces of dust grains than expected from the deuterium/hydrogen ratio in the Universe. Since water molecules first form on dust grains, this high ratio of heavy to normal water acts like a chemical fingerprint that allows us to trace the subsequent journey of water. In other words: by measuring the relative amount of heavy and normal water in different environments – molecular clouds, proto-planetary discs, comets, planets – we can figure out if water molecules were altered along this trail.
Single or double?
Heavy water comes in two flavours so to speak: semi-heavy water, or HDO, where only one of the two hydrogen atoms is deuterium, and doubly-deuterated heavy water, or D2O, which contains two deuterium atoms.
Two years ago, a group of astronomers detected HDO in the proto-planetary disc around V883 Ori, a proto-star 1300 light-years away. Using the ESO partnered Atacama Large Millimeter/submillimeter Array (ALMA), they measured an abundance of HDO very similar to that of comets in our Solar System. Assuming that this proto-planetary disc is not too different from the one that birthed our Solar System, this finding provided key evidence that the water we see today is the same pristine water formed in the Sun’s parent cloud.
But this wasn’t definitive proof, because HDO molecules can still be destroyed by the star’s radiation and then reform, thus somewhat blurring its past history. This is much less likely to happen with D2O, which is thus a more robust tracer of water’s history.
Now, the same team of astronomers have detected D2O in the disc around the V883 Ori star using ALMA. Their results, published in a Nature Astronomy paper led by Margot Leemker, an astronomer at the University of Milan, Italy, show a high abundance of D2O. This abundance is similar to that found in molecular clouds and one comet [1], suggesting that the water we find in the current Solar System is the same water that existed long before the Sun had formed.
How water actually makes it to planets, including Earth, is a complex issue that needs more research, but this new finding is a key piece in the puzzle. So the next time you enjoy a refreshing glass of water, take a second and consider this: at least some of the water you are tasting not only passed through the dinosaurs but in fact predates the Earth itself and even the Sun!
Notes
[1] While the abundance of HDO has been measured in several comets, we only have D2O measurements in one.
Biography Amy Briggs
Amy is a recent science communication graduate from the Australian National University. Before coming to learn from the COMMS team at the ESO, she worked for the Australian Academy of Technological Sciences and Engineering (ATSE) as a Communications Coordinator and as an Editorial Coordinator for the youth magazine Careers with STEM.
She has also written for the Sunday Space column in The Canberra Times, ABC Science, and the CSIRO’s Double Helix magazine.
She has a passion for storytelling, reading, and unihockey.
Biography Malika Duffek
While pursuing her Bachelor's degree in Astrophysics, Malika discovered a deep passion for Science Communication. She joined the Mobile Planetarium team at her institute in Vienna, where she brought the wonders of the universe to life for schoolchildren across the region. Since then she has continued to explore further areas of outreach. She went on to complete her Master's in Astrophysics and broaden her expertise as a Science Communication Assistant at the University of Vienna. Today, she is part of ESO, excited to learn from an international team and continue expanding her skills across diverse fields of science outreach and engagement.



